Water, a necessary solvent for life is all around us but only about 1.2% of it is fresh water that can be used for drinking.1 Without it we die, and that death can be agonizing and slow especially if you are drinking unsafe water and get infected by parasitic organisms or bacteria and viruses which cause diarrhea that causes you to actually expel your own life liquid over several days until there is nothing left in you. The irony is that that same liquid, if not properly sanitized, will become a transmitter of infection to others. You do not want to die because of diarrhea, or for that matter, you don’t want anyone to die from it.
So, the United Nations has set itself a goal that by 2030 access to safe drinkable water would be universal.2
Of all the water on Earth, more than 99 percent of Earth's water is unusable by humans and many other living things. It seems extraordinary that the water that supports all terrestrial, as well as aquatic, life on our planet is actually so scarce.
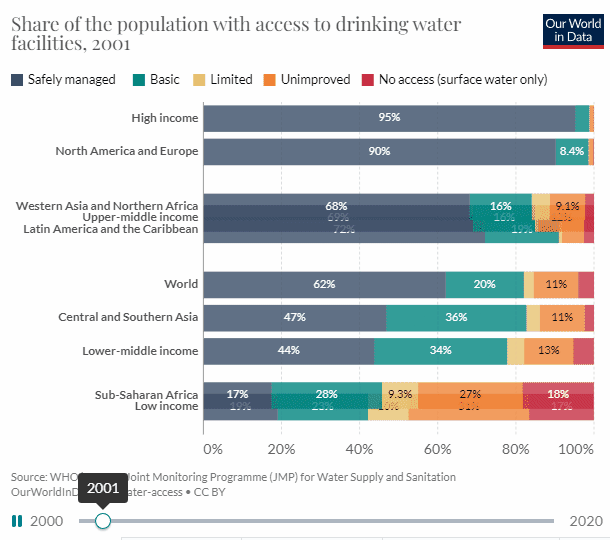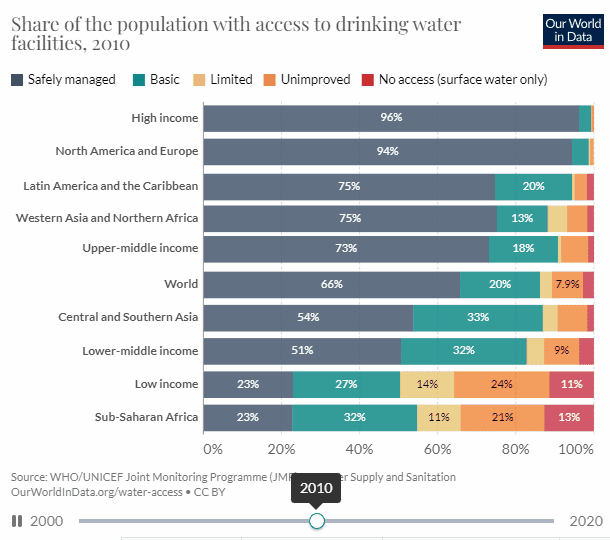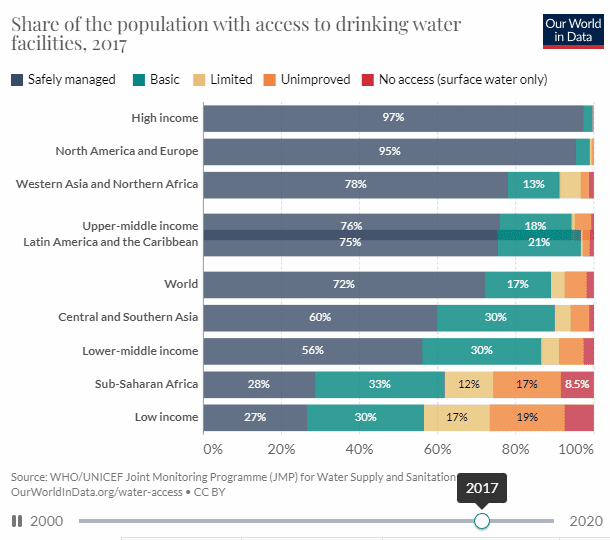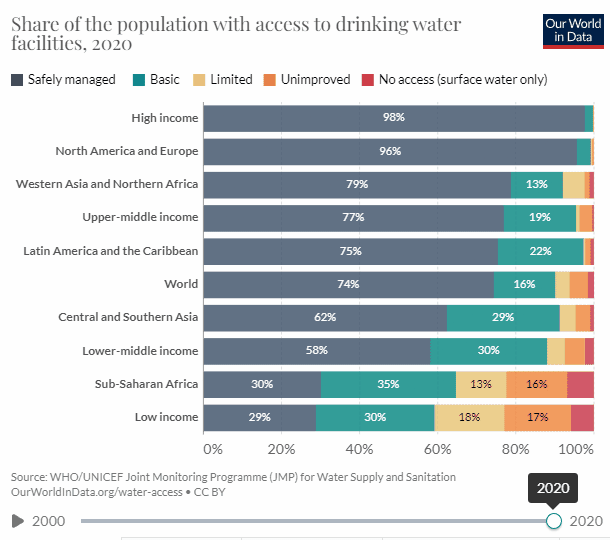
There is good news, well mixed at best. According to UN data, access to potable water has increased by 12.54% from 2000, and 61.73% to 74.27% in 2020, which is nothing to sneeze at. But to reach universal access within 10 years we need a 13 percentage point increase every five years.
When looking at the chart we can see that the biggest issue is Sub-Saharan Africa.
There are many ways we access our drinking water but in most cases, it’s via wells. The average cost to bore a well is around $200 per meter and depending on where you are in the world that could add up to quite a lot if you need to bore more than 10m down to find an aquafer. That’s only the borewell, not the casings, pipes, pumps, etc. And, that’s not to mention more classically dug-up wells that really don’t have a cap on how much they can cost. They can be free if you dig them yourself or cost tens of thousands to dig and make them structurally sound.
According to March 2022 exchange rates, the average monthly pay in Africa is around 758 USD (US dollars), and that varies wildly depending on the country. The average salary per month in Sudan is $61 while in Kenya it’s $1,089. Also note that this is an average salary, if looking at the median it paints a different picture. A median salary for a country indicates that 50% of people earn less than the median salary, and 50% of people earn more. Most of Africa is below $500 of median monthly income. This is roughly fifteen times lower than typical incomes in the United States and the United Kingdom, which are 7,900 USD and 7,795 USD respectively.
So, that’s not really a solution. A solution would be something ordinary working people could afford.
I remember a concept from several decades ago where a tripod-looking red upside-down water drop-looking thing had a camel hair underside, where usually the top of the waterdrop would be. I can’t find it anywhere online anymore, could be some kind of Mandela effect… anyway.
While doing my rounds of reading papers I found a “Review of sustainable methods for atmospheric water harvesting” from the International Journal of Low-Carbon Technologies published in 2020. So if you want to dive in deeper, that’s your starting point.
Contrary to popular belief, there is no such thing as a true desert. Even in the most arid places, there is water in the air. Its percentage and accessibility could be extremely low but it’s still there. It’s all part of the water cycle.
There are two types of moisture in the air.
Fog, or visible water droplets or ice crystals that are suspended in the air at or near the Earth’s surface. It normally occurs due to added moisture in the air or falling ambient air temperature. And then there is water vapor. While fog is visible to our naked eyes, water vapor is invisible and is generated by the evaporation of liquid water or the sublimation of ice. When water vapor condenses on a surface that is below the dew point, dew is formed.
While collecting fog is rather simple as the amount of water in the air when fog is concerned is high, collecting water vapor is much more difficult.
The fog-collecting method is straightforward. Get a large surface area where water can condense and you get water out of air. It’s very simple and cheap, comprising of a mesh exposed to the atmosphere over which the fog is driven by the wind.
The mesh is usually cheap Anti-Hail and Agricultural Shade Net mesh made from High-Density polyethylene (HDPE). It’s really cheap as one square meter costs around $0.5. A typically 8m long and 6m high mesh with coverage of about ∼48 m2 can produce 150L to 750L of water a day depending on where it’s located.
HDPE is a strong and durable material but it still can be damaged. A good substitute or more accurately said an addon to HDPE mesh is a stainless mesh. It’s more durable but its surface area is smaller so it’s usually only used as reinforcement of HDPE mesh. Steal mesh can be used as a substrate on which HDPE mesh is tied, or more costly, HDPE can be woven throughout the steel mesh.
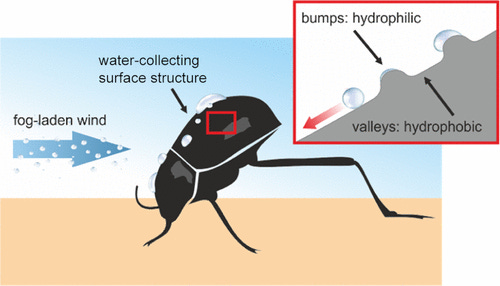
What we are doing here is trying to mimic in some sense the Stenocara gracilipes that survive in the Namib desert by collecting water although the annual rainfall is only 12 mm. The surface of the beetle’s back is covered with a random array of smooth hydrophilic bumps and microgrooves 0.5 mm in diameter and arranged at 0.5–1.5 mm intervals. These bumps on the forewings are a few in microns in size allowing water to condense and trickle directly to their mouth. But in this case, it would be more accurate to say that we are mimicking the spider web but its main purpose is not to gather water.
The secret of its success is that the bumps are hydrophilic, they attract water, and when a sufficient amount collects to break off due to its own weight it slides off down and keeps sliding as unlike the bumps the rest of its body is hydrophobic.
But, not all places have fog, and as it turns out it is more scarce than I imagined.
Only limited number of places experience environmental conditions whereby the temperature of moist air could naturally drop below its saturation temperature thus form fog. Not surprisingly therefore, on a global scale, fog is reported to be even less accessible..
Compared to fog, water vapor is ubiquitous in the atmosphere, and is condensed by cooling. The condensation process is more thermodynamically complicated than fog harvesting and an air temperature needs to be reduced significantly to bring it to below its dew point and to condensate.
There are three categories of approaching this issue. We have a passive or radiative cooling condenser, then solar-regenerated desiccant and water harvesting from the air using active cooling condensation technology.
“The principle of radiative cooling condenser is very simple. Inspired from dew formation on plants in the morning, the formation of dew is driven by radiation phenomena of the surface of the materials. The formation of the dew is physical and determined by the surface cooling without additional energy, and the most important element being the power gradient between the condenser outgoing radiative power and the sky radiative power which is presented by the Stefan–Boltzmann law.”
Essentially this works because everything gives off heat, and the Stefan-Boltzmann law helps us understand this. This law tells us how much heat or energy something gives off based on its temperature.
So, this cooling condenser works by making sure it gives off more heat into the sky than it absorbs from the surroundings. This way, it can keep getting cooler and cooler without needing any extra power. It's a bit like how your body cools down when you're sweating on a hot day – the sweat evaporates, taking away heat and making you feel cooler. In this case, instead of sweat, it's the heat going into the sky in the form of infrared radiation, and that’s how it cools things down.
In 2000, Alnaser WE, and Barakat A. in their paper on “Use of condensed water vapour from the atmosphere for irrigation in Bahrain” tested several materials to check which is best as a surface for condensing water vapor. “Three condensation surfaces have been tested; aluminum, glass, and polyethylene foils. The average quantity of dew collected on these surfaces was 1.3, 0.8, and 0.3 kg/m2 per h, respectively.” Take these results with a huge amount of salt as their research is suspect in my eyes and contains misleading information as the best passive dew collector manages only 2.5 l/day/m3 which is way lower than what they say they managed.
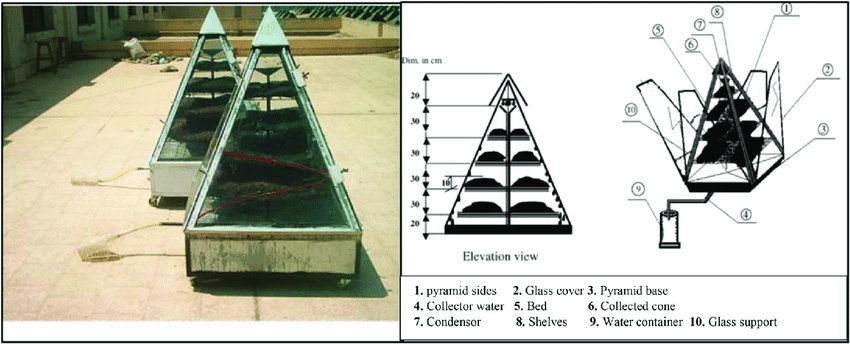
Studies also showed that the shape of the surface is also important. There are many different approaches, from Glass pyramid collector, Corrugated surface, Trapezoidal prism, Solar glass desiccant box type system, and so on.
Now, there are other systems that can be used to collect water vapor from the air but they are all active systems that use all sorts of thermoelectric generators (TEG) to cool down the collection surfaces. In day-to-day use, we call them dehumidifiers. As they consume power they are not in the scope of this post. But what is, is absolutely brilliant video and research done by NightHawkInLight over on his YouTube channel where he describes his methods of making radiative sky-cooling paint with common grocery and hardware store items. This paint when properly applied can allow for clean, electricity-free air conditioning to several degrees below ambient air temperature in direct sunlight. Combining his paint with an aluminum surface could be amazing for water vapor collection. Until I get to testing that here is his recipe and a link to his video.
NightHawkInLight's super CaCO₃ micro-sphere pigment recipe:
Sodium Carbonate (washing soda) ......... 20g dissolved in 200 mL water
Calcium Chloride (painter's desiccant) ... 10g dissolved in 100 mL water
Citric Acid (used for canning) .................. 3g dissolved in 30 mL water
1. Combine Calcium Chloride and Citric Acid solutions into one container.
2. Adjust the temperature of the liquids to be between 10-20°C (50-70°F).
3. Pour the Calcium Chloride/Citric Acid solution into a mixer and begin stirring.
4. Pour the Sodium Carbonate solution into the mixer and begin a timer.
5. Allow solution to mix for 1 minute.
6. Turn off the mixer and pour the liquid into a separate container.
7. Wash the mixer with vinegar and water to prepare for the next batch.
**Make 3 total batches following steps 1-7, each time allowing the solution to mix for 1 minute. To save time you can make large quantities of the starting solutions all at once, chill all of it to 10-20°C, and then measure out enough for individual batches by volume just prior to mixing.
8. Make a fourth batch, mixing for an increased time of 5 minutes.
***Optional: Make a fifth batch at 1/3 scale, mixing for an increased time of 8-10 minutes.
9. Allow 20-60 minutes for the pigment to settle out of all batches.
10. Pour the water off the top of the settled pigment and refill containers with water.
11. Repeat settling and pouring off water 1-2 more times.
12. Cut a rectangular opening in the bottom of a disposable bread tin.
13. Line the tin with a paper towel and a sheet of white printer paper.
14. Pour the pigment solutions into the tin, using the paper as a filter.
15. Pour extra distilled water over the pigment to wash it.
16. Once filtered, place the whole tin into an oven to dry at 100°C (212°F).
17. Collect pigment, breaking up clumps in a blender if needed.
It is important to note that atmospheric water, is not safe to drink. It contains biological contamination from spores and bacteria of various sources. This contamination is usually unavoidable because dew and fog condensers are placed in an open environment. This means that dew water must be disinfected for use as a beverage.
https://education.nationalgeographic.org/resource/earths-fresh-water/
https://www.un.org/sustainabledevelopment/water-and-sanitation/