Graphene made a big impact since it was isolated and there are tens of thousands of research papers published yearly, so it's impossible to provide a general overview, but I’ve been lurking around graphene for a long time and this is a summary of the most important information so far.
Graphene is currently where aluminum was in the 19th century when it was used for luxury eating utensils that cost more than gold ones. Even when manufacturing costs fell it remained a niche material until the invention of the airplane, which uniquely required aluminum’s specific combination of strength and weight to make commercial flight viable. Graphene has so many uses that it will require an entire new post for just some of them. The present commercial price of graphene is $67,000 to $200,000 per ton depending on layer count and contaminants.
Graphite is, not to be confused with graphene, a bulk material consisting of many graphene layers and, as such, an exposed surface of graphite is a model graphene surface on a graphite substrate. A graphene layer is one atom thick effectively making it the first true 2D material or as much as any material can be in our 3D world and its properties are exceptional. It is an allotrope of carbon, just a different rearrangement of carbon atoms found in almost anything. Most of our trash if not all of it is made out of carbon. Paper, oil, organic matter, plastic, old tires, all can be used for the production of graphene. Not all methods can use trash as feedstock as up to now the most used raw source for production was naturally-occurring graphite, but such mines are few and "China has a stranglehold on natural graphite supply," says Simon Moores of Industrial Minerals, "This, together with a generation of under-investment in mines around the world, is creating a very tight supply situation" of high-quality graphite.
Although scientists knew one atom thick, two-dimensional crystal graphene existed, and I remember learning about the structure of graphite in elementary school, no one had worked out how to extract it from graphite. That was until it was isolated in 2004 at The University of Manchester. Professor Andre Geim and Professor Kostya Novoselov won the Nobel Prize in Physics in 2010 for their work. The story of how they isolated a single layer of graphene is amazing in its simplicity and genius. They used a method of mechanical exfoliation or in plain talk, they used a piece of sticky tape.
A fresh piece of Scotch tape, simple common placed sticky tape where the adhesive side was pressed onto the Highly Ordered Pyrolytic Graphite ore. The tape is then gently peeled away with thick shiny layers of graphite attached to it.
The part of the tape with layers from the HOPG was refolded upon a clean adhesive section of the same piece of the tape and then the tape was unfolded. This process is repeated several times until the end of the tape is no longer shiny but becomes dark/dull and gray. These graphite layers on the tape were transferred onto the surface of the Si/SiO2 wafers by gently pressing them onto the tape for some time and then peeled off, so they could be examined.
The process is simple and safe and a few layers of graphene can be easily obtained. But as simple as this method is it can not yield a large amount of the material.
Graphene was found to be harder than diamond in certain conditions, and 200 times stronger than steel with a surface area of 2,630 m2/g making it an ideal material for making batteries. It is also a superconductor even at room temperature which conducts electricity more easily and more quickly than copper, and a million times the current density. It conducts heat of 5,000 W / meter Kelvin, much better than any other carbon structures such as carbon nanotubes, graphite, and diamond.
Single-layer graphene absorbs only 2.3% of the incident light, which makes it more transparent than anything else and a perfect candidate as a substrate for transparent screens. It is the most impermeable atomic layer, leaving no liquid or gas through it, it is hydrophobic, fire retardant, lubricant, and protects from corrosion, electromagnetic waves, and UV.
Graphene is not rejected by our bodies making a computer-human-computer interface a real possibility or any other application where organic tissue needs to be connected to something else. Maybe even nerve repair or augmentation. Deus Ex here we come.
Now, with all those amazing properties why is graphene still a sideline material? Well, it is kind of hard to produce. This contradicts the earlier stated proposition that graphene is easy to produce. It is but if the quality is expected then not so much. There are two major ideas or paths when producing graphene. The first one is if we want to produce a dispersion material like ink pigment, and the second is the production of a pristine monolayer sheet of graphene. The second one, the sheet, is extremely hard to get especially noncontaminated large sheets of it. First, the dispersion material is easy to produce from high-quality raw graphite but such sources are few and such material is expensive.
Many different terms and conflicting notations are used even in the scientific community. No single notation has yet prevailed and this creates a lot of noise. The terms of material under the name Graphene mean a honeycomb sheet with a single atom thickness. However, it seems to have slightly evolved in usage to mean "graphite thin enough to show peculiar properties". Anything above 10 sheets thick is electronically sufficiently similar to bulk graphite that it can be called such and is not considered graphene.
Since its discovery and realization of its promise as super material Graphite has been named a "supply critical mineral" and a "strategic mineral" by the United States and the European Union. EU started in 2013 The Graphene Flagship Organization where Future and Emerging Technology is cultivated with a budget of €1 billion. The Graphene Flagship represents a new form of joint, coordinated research forming Europe's biggest-ever research initiative. The original research mandate was to last until 2020 but was further funded by The European Commission with a €150M grant from 2020 to 2023.
The Graphene Flagship is tasked with bringing together academic and industrial researchers to take graphene from the realm of academic laboratories into European society in the space of 10 years, thus generating economic growth, new jobs and new opportunities.
The core consortium consists of over 150 academic and industrial research groups in 23 countries. In addition, the project has a growing number of associated members that will be incorporated in the scientific and technological work packages from the Horizon 2020 phase.
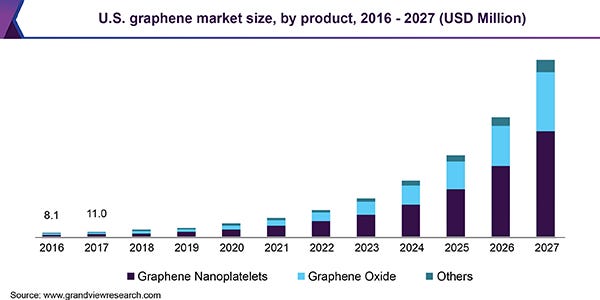
This lack of high-volume production and high price resulted in the global market being only $78.7 million in 2019. But all of that changed in 2020 with the discovery of flash graphene. Now, expectations are that with high-volume production discovered the global graphene market will reach $2.8 billion in 2027. That is a big jump and all thanks to …
Flash Graphene
The “flash” process now officially called the flash Joule heating process for obtaining turbostratic graphene was discovered in a lab of chemist James Tour at Rice University in 2020 by his graduate student Duy X. Luong. The process is simple in itself. Ground-up organic material is placed between two electrodes and zapped with so much current that it instantly goes from room temperature to over 2700C. This boils and breaks all noncarbon elements out and if the zap time is short enough the leftover material doesn’t have time to rearrange itself into anything else than the simplest form of carbon can take and that’s graphene. The released gas can be used and processed to get to a myriad of additional interesting elements so this process does not only provide graphene but a lot of other interesting industrial gases.
As reported in Nature, flash graphene is made in 10 milliseconds by heating carbon-containing materials to 3,000 Kelvin (about 5,000 degrees Fahrenheit). The source material can be nearly anything with carbon content. Waste food, plastic waste, petroleum coke, coal, wood clippings and biochar are prime candidates, Tour said. “With the present commercial price of graphene being $67,000 to $200,000 per ton, the prospects for this process look superb,” he said.
The Rice lab flashed tire-derived carbon black and found about 70% of the material converted to graphene. When flashing shredded rubber tires mixed with plain carbon black to add conductivity, about 47% converted to graphene. Elements besides carbon were vented out for other uses.
The electrical pulses lasted between 10 milliseconds to 300 milliseconds and 1 second. The lab calculated electricity used in the conversion process would cost about $100 per ton of starting carbon.
Laser Induced Graphene
A similar method is laser-induced graphene where Kapton tape is heated using a laser source to produce graphene on its surface. It’s not a precise method and no control of quality is maintained but for certain applications that is good enough.
Other “Older” production methods
Electrochemical Exfoliation
The electrochemical exfoliation method is an eco-friendly method for producing high quality graphene.
The electrochemical exfoliation of graphite is due to the ions present in the solution that permeate between the layers making them easier to separate using an electrical current.
The choice of electrolyte is based on the requirement of the oxidizing environment and more importantly on the size of the intercalating ion. The setup includes two electrodes one of them being graphite or HOPG the other can be Cu, Pt, or HOPG/graphite. A voltage of +/- 10V is applied generally for an unequal period of time. The intercalating ion in the solution exfoliates the graphite into few-layer graphene or FLG by penetrating in between the sheets due to the applied voltage.
The need for negative voltage is to bring the intercalated ions back into the solution along with the sheets, hence the need for unequal application time for the voltages. The electrolyte used is often diluted with DI water and then at the end of the process, the solution is taken for centrifugation to separate the graphite particles from graphene.
The solution can also be subjected to ultra-sonication for enhancing the exfoliation and some stabilizers can also be used for the solution.
The final solution is either dried or taken directly for characterization.
Advantages:
High yield.
Scalable to industrial level.
Easy to operate and relatively a faster approach.
Eco-friendly.
FLG can be easily obtained.
The graphene obtained can be functionalized depending upon electrolyte and hence can be more compatible with certain organic compounds or polymers.
Limitations:
Needs a source of existing Highly Ordered Pyrolytic Graphite.
The impurities may be present in the form of unwashed salts in between the graphene layers. This may affect the conductivity of graphene.
The thickness control is not as promising as in the epitaxial graphene growth or the CVD process.
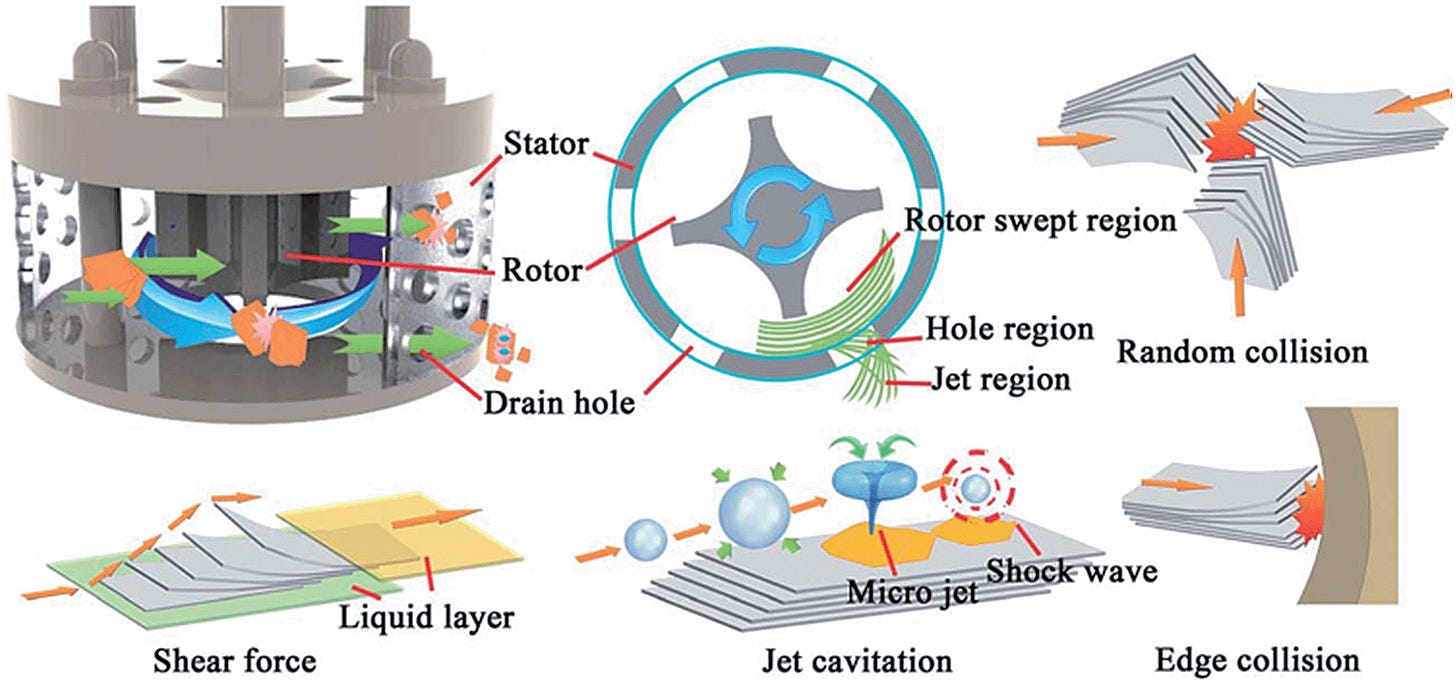
Mechanical Shear Exfoliation
Defect-free few-layer graphene nanosheets can be produced using high-shear mixing in rotor-stator mixers or rotating blade mixers of quality HOPG material. This method is scalable and can be performed in large volumes without loss of product quality or degree of exfoliation with production rates up to 5.3 g/h.
This process was licensed, patented, and led to the commercialization of the graphene ink product Elicarb® by Thomas Swan & Co. Ltd. (100ml / 1g/l @ £125)
Shear exfoliation can also be used as a pretreatment or posttreatment technique in other processes, the same as sonication.
CVD (Chemical Vapour Deposition)
CVD is used to produce large single-layer sheets. Large sheets are still small by our macro world but still useful for making sensors, filters, and alike. If macro scale sheets can be produced then transparent screens or HUD on glasses or lenses become so much closer to reality.
The carrier gases are combined in a reaction chamber which is maintained at a certain temperature and pressure. The reaction occurs on the substrate on which one of the products is deposited and the by-products are pumped out. The substrate is usually a transition metal (Ni/Cu) or some ceramic such as glass. The selection of substrate depends upon the feasibility of transferring the graphene onto the required material.
The gases used are generally Methane (source of carbon) Hydrogen and Argon are also used along with methane as the reaction stabilizes and enhances the film uniformity.
Advantages:
High-quality, impervious, and harder graphene is obtained.
Producing large domains of graphene is easy.
High growth rates are possible.
Good reproducibility.
Limitations:
High temperatures (greater than 900 °C) leads to wrinkled graphene.
Complex process.
Production of corrosive and toxic gases.
Difficulty in controlling the thickness in some cases (number of layers).
Difficulty in transferring the film to another surface (exfoliation).
Difficulty in achieving the uniform deposition of the carbon.
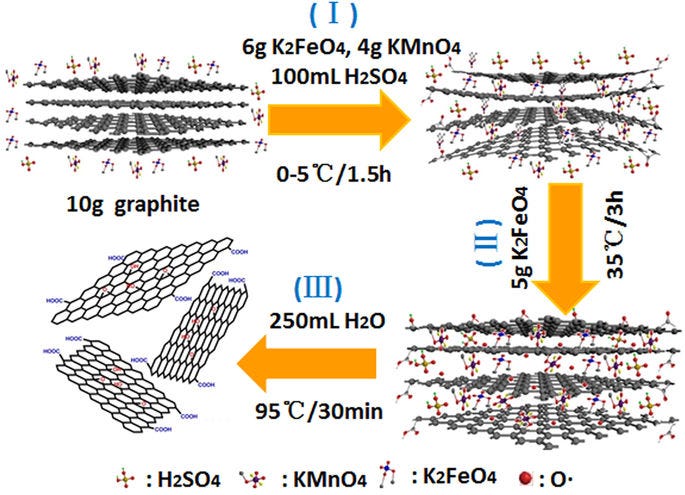
Hummers Method (modified)
The Hummers method is used for producing graphene by oxidizing graphite to graphite oxide (GO) by using suitable oxidizing agents such as KMnO4. The Graphene Oxide so produced is again then chemically reduced to get graphene.
The modified Hummers method introduces a way to get a more stable GO colloidal solution. Ultra-sonication is used for stabilizing the GO solution and enhancing the exfoliation in the GO solution.
The process is rather complicated and has several steps. the first step is Oxidation where natural graphite flake is mixed with a strong acid such as H2SO4/HNO3 followed by continuous stirring in an ice bath. Then KMnO4 is added and stirred at room temperature. The solution is kept overnight after adding DI water and H2O2 then Centrifugation is used for dilution until the pH is around 7. Ultra-sonication is carried out to get monolayer GO.
The next step is Reduction. The addition of certain reducing agents such as hydrazine or NaBH4 is made to the measured solution. The attached functional groups are removed and to enhance the exfoliation certain polar aprotic solvents can be used along with organic compounds. Although thermal reduction gives better quality graphene but has its own disadvantages.
And then post-treatment where the solution is filtered and washed with DI water until neutrality. Finally, the product is dried and ground, and sent for characterization tests.
Advantages:
High yield.
Scalable to industrial level.
Limitations:
Needs source of existing Highly Ordered Pyrolytic Graphite
The defects on graphene sheets are inevitable.
The process is time-consuming and can be laborious.
The thickness control is not as promising as in the epitaxial graphene growth or the CVD process.
Explosive / Toxic reactive environment / Byproducts